To solve this
problem, remember that a gas confined inside a cylinder equipped with a piston
can do work on the piston, and this work can be calculated by multiplying the
pressure by the change in volume. If the volume doesn't change, no work is
done. If the pressure stays constant while the volume changes (an “isobaric
process”), the work done is easy to calculate – you just need to calculate the
initial volume and the final volume, take the difference and multiply it by the
pressure.
As an aid in
calculating the work done, it's a good idea to draw a pressure-volume graph
(with pressure on the y axis and volume on the x-axis). If a system moves from
one point on the graph to another and a line is drawn to connect the points,
the work done is the area underneath this line.
The P-V graph for this
isobaric problem looks like this:
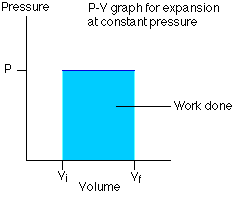
In this
problem, we know that P = 413 kPa, so all we have to
do is calculate the initial volume (Vi) and the final volume (Vf), take the difference and multiply the
difference by the pressure. In other words, W = P(Vf – Vi).
To calculate
the initial and final volume, we need to use the formula PV = nRT, where n is the number of moles, R = 8.314 J·K−1·mol−1, and T = the temperature in Kelvin. Another
way of writing the equation is V = (nRT)/P. In this
problem n = 1290 moles, since there are approximately 1290 moles
in 22 kg of ammonia (one mole of ammonia weighs approximately 17.03 grams), and
the initial value of T = 311.15 degrees Kelvin.
Vi = (nRTi)/P
= (1290 mol * 8.314 J·K−1·mol−1 * 311.15° K )/413
kPa = 8080.15 Liters
Vf = (nRTi)/P
= (1290 mol * 8.314 J·K−1·mol−1 * 373.15° K )/413
kPa = 9690.21 Liters
Now we can easily
calculate the work done:
W = P(Vf - Vi) = 413 kPa (9690.21 L - 8080.15 L) = 413 kPa
* 1610.06 L = 664,954.78 J = 665 kJ